- Your cart is empty
- Continue Shopping
BCHCT-135 SOLUTIONS, PHASE EQUILIBRIUM, CONDUCTANCE, ELECTROCHEMISTRY & FUNCTIONAL GROUP ORGANIC CHEMISTRY-II in English Solved Assignment 2021-2022
₹40.00
BCHCT-135 SOLUTIONS, PHASE EQUILIBRIUM, CONDUCTANCE, ELECTROCHEMISTRY & FUNCTIONAL GROUP ORGANIC CHEMISTRY-II
Solved Assignment 2021-2022
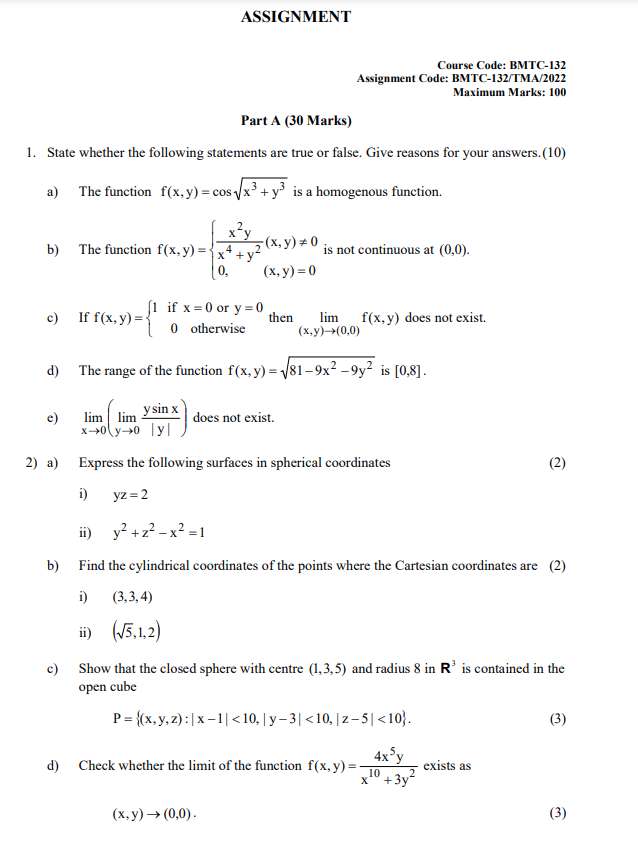
ASSIGNMENT
Solutions, Phase Equilibrium, Conductance, Electrochemistry & Functional Group Organic
Chemistry-II
Core Course in Chemistry
Course Code: BCHCT-135
Assignment Code: BCHCT-135/TMA/2021-2022
BCHCT-135 SOLUTIONS, PHASE EQUILIBRIUM, CONDUCTANCE, ELECTROCHEMISTRY & FUNCTIONAL GROUP ORGANIC CHEMISTRY-II
Solved Assignment 2021-2022
ASSIGNMENT
Solutions, Phase Equilibrium, Conductance, Electrochemistry & Functional Group Organic
Chemistry-II
Core Course in Chemistry
Course Code: BCHCT-135
Assignment Code: BCHCT-135/TMA/2021-2022
| Title Name | BCHCT-135 Solved Assignment 2021-2022 |
| University | IGNOU |
| Service Type | Solved Assignment (Soft copy/PDF) |
| Course | BSCG |
| Language | ENGLISH |
| Semester | 2021-2022 Course: B.SC(G) CBCS |
| Session | Session: July 2021- January 2022 |
| Short Name | BCHCT-135 |
| Assignment Code | BCHCT-135/TMA/2021-2022 |
| Product | Assignment of BSCG 2021-2022 (IGNOU) |
| Submission Date | Valid from 1st July, 2021 to 30th June, 2022 |
| Price | RS. 50 |
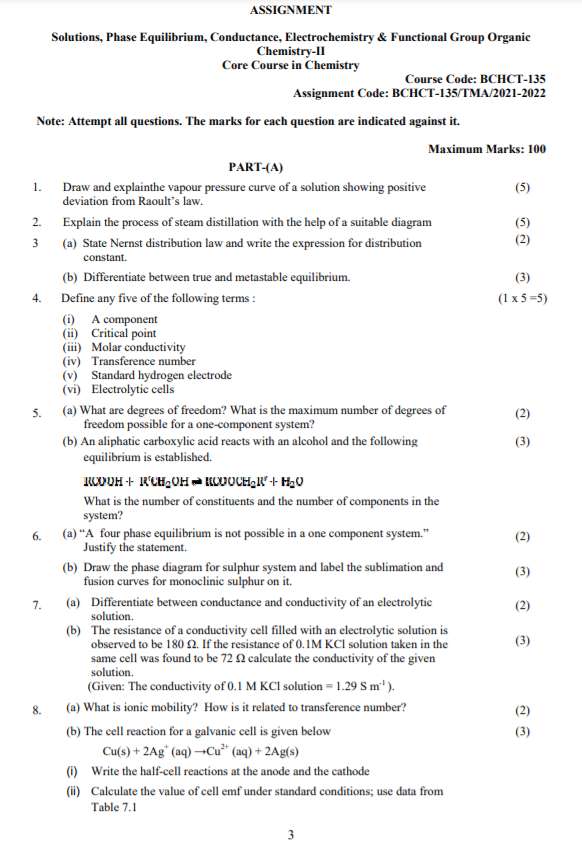
PART-(A)
1. Draw and explainthe vapour pressure curve of a solution showing positive
deviation from Raoult’s law.
(5)
2. Explain the process of steam distillation with the help of a suitable diagram (5)
3 (a) State Nernst distribution law and write the expression for distribution
constant.
(2)
(b) Differentiate between true and metastable equilibrium. (3)
4. Define any five of the following terms :
(i) A component
(ii) Critical point
(iii) Molar conductivity
(iv) Transference number
(v) Standard hydrogen electrode
(vi) Electrolytic cells
(1 x 5 =5)
5. (a) What are degrees of freedom? What is the maximum number of degrees of
freedom possible for a one-component system?
(2)
(b) An aliphatic carboxylic acid reacts with an alcohol and the following
equilibrium is established.
What is the number of constituents and the number of components in the
system?
(3)
6. (a) “A four phase equilibrium is not possible in a one component system.”
Justify the statement.
(b) Draw the phase diagram for sulphur system and label the sublimation and
fusion curves for monoclinic sulphur on it.
(2)
(3)
7. (a) Differentiate between conductance and conductivity of an electrolytic
solution.
(b) The resistance of a conductivity cell filled with an electrolytic solution is
observed to be 180 Ω. If the resistance of 0.1M KCl solution taken in the
same cell was found to be 72 Ω calculate the conductivity of the given
solution.
(Given: The conductivity of 0.1 M KCl solution = 1.29 S m-1 ).
(2)
(3)
8. (a) What is ionic mobility? How is it related to transference number? (2)
(b) The cell reaction for a galvanic cell is given below
Cu(s) + 2Ag+
(aq) →Cu2+ (aq) + 2Ag(s)
(i) Write the half-cell reactions at the anode and the cathode
(ii) Calculate the value of cell emf under standard conditions; use data from
Table 7.1
(3)
4
(iii) Will the reaction be spontaneous as written?
9. (a) Differentiate electrolyte concentration cell and electrode concentration
cells.
(2)
(b) The cell reaction for a galvanic cell is given below
Cu(s) + 2Ag+
(aq) →Cu2+ (aq) + 2Ag(s)
(i) Write the half-cell reactions at the anode and the cathode
(ii) Calculate the value of cell emf under standard conditions; use data from
Table 7.1
(iii) Will the reaction be spontaneous as written?
(3)
10. (a) Calculate the time for which a current of 0.65 A be passed through
molten magnesium chloride to get 5 g of magnesium.
(Given: Mm(Mg) =24.25 g mol -1)
(3)
(b) Draw a labelled schematic graph of a conductometric titration between a
weak acid and a strong base.
(2)
PART-(B) (50)
11. Explain Fischer esterification of carboxylic acids giving its mechanism. (5)
12. How would you prepare the following?
(i) Ethanoic anhydride using ketene (2)
(ii) A tertiary alcohol starting from an ester (3)
13. (a) What are lactams? Write the structures of -butyrolactam and -valerolactam and give their
IUPAC names.
(3)
(b) Draw the enantiomers of N-methylethanamine. (2)
14. Explain the nitrosation reaction of secondary and tertiary amines giving suitable reactions. (5)
15. How can you prepare 1,3,5-tribromobenzene? Why it cannot be prepared by bromination of
benzene ?
(5)
16. How will you synthesise valine using Gabriel phthalimide synthesis? Explain giving the sequence
of reactions involved.
(5)
17. Discuss ninhydrin test giving the reactions involved. (5)
18. Explain Edman degradation giving the reactions involved in it. Give the advantage of this method. (5)
19. (a) Explain the formation of methyl glycoside of - and - D-glucose. Also give their structures. (2)
(b) Write the reactions involved when -D-glucose is treated with excess of acetic anhydride in the
presence of pyridine.
(3)
20. (a) Differentiate between reducing and non-reducing sugars giving one example of each type. (2)
(b) Write the reaction of formation of maltose from its monomers giving appropriate structures with
proper labeling.
BCHCT-135, BCHCT 135, BCHCT135












Reviews
There are no reviews yet.